Abstract
Background: Poly (ADP-ribose) polymerase (PARP) enzymes are involved in repair of single-strand DNA breaks through base excision repair pathways. Inhibitors of PARP are approved for the treatment of BRCA1/2-mutant malignancies. We have previously demonstrated (Portwood et al, ASH 2019 abstract) that PARP inhibitors can synergistically enhance the activity of DNA-damaging agents in preclinical models of human acute myeloid leukemia (AML). Talazoparib (Tala) is a selective PARP inhibitor which exhibits potent inhibitory effects against multiple human AML cell lines. Gemtuzumab ozogamicin (GO) is an CD33 antibody drug conjugate approved for treatment of patients (pts) with AML. We hypothesized that the combination of Tala + GO would result in improved efficacy as compared with the historical response of GO monotherapy in pts with relapsed or refractory (R/R) AML.
Study Design: This open-label multi-center phase 1b study evaluated the safety, tolerability, and preliminary response rates for Tala + GO in adult pts with CD33+ R/R AML. In the dose escalation portion, pts will be treated with Tala (dosed at 0.5, 0.75, or 1 mg orally daily) in combination with fixed dose GO (3 mg/m 2/day on days 1, 4, and 7, capped at one 4.5 mg vial) using a standard 3+3 design. The dose limiting toxicity (DLT) window is 28 days. After determination of DLTs and establishment of a recommended phase 2 dose, additional pts are planned for an expansion cohort.
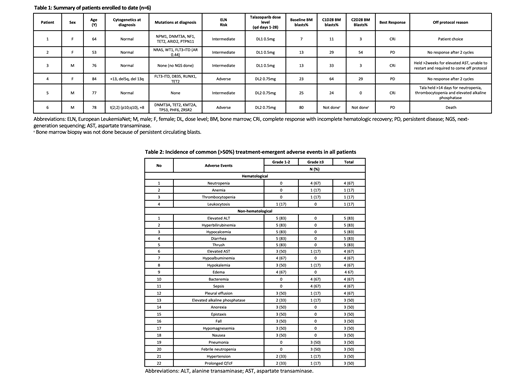
Results: This trial was activated in July 2020 and is registered at ClinicalTrials.gov (NCT04207190). As of August 2021, 6 pts have been enrolled, 3 each at Tala dose levels of 0.5 and 0.75 mg daily, respectively. Median age is 77 (range, 53-84) years with 3 (50%) women (Table 1). Median prior lines of therapy were 3 (range, 1-7), and 2 pts had received prior allogeneic transplant. Four pts had intermediate European LeukemiaNet risk disease at diagnosis. Five pts had next-generation sequencing at diagnosis (Table 1). One pt had p53 mutant AML (1/5, 20%), and two pts had FLT3 mutations (2/6,33%).
To date, there have been no DLTs. The most common adverse events (AEs) of any grade included elevated alanine transaminase, hyperbilirubinemia, hypocalcemia, diarrhea, and oral thrush (83% each) (Table 2). Grade ³3 hematological AEs were common and related to Tala + GO. These consisted of neutropenia (n=4, 67%), anemia (n=1, 17%), and thrombocytopenia (n=1, 17%). The most frequent non-hematological grade ³3 AEs were bacteremia (67%), sepsis (67%), febrile neutropenia (50%), and pneumonia (50%). All were considered unrelated to Tala (Table 2).
Three pts (50%) achieved a best response of complete response with incomplete count recovery (CRi) after 2 cycles. Two of these 3 pts were dosed at Tala 0.5 mg oral daily. All 6 pts are currently off protocol. Two had no response after 2 cycles, 1 died from persistent diease, 1 had persistently elevated AST, 1 was pt choice, and 1 died of pneumonia/respiratory failure. To date, a protocol defined maximally tolerated dose (MTD) has not been identified.
Conclusion: This open-label multi-center phase 1b study is evaluating the safety, tolerability, and preliminary response rates of Tala + GO in adult pts with relapsed/refractory CD33+ AML. To date, 6 pts have been enrolled at the first two dose levels (Tala 0.5 mg po daily + GO, Tala 0.75 mg po daily +GO). No DLTs or MTD have been identified. The overall response rate after 2 cycles of therapy was 50% (n=3, CRi). A recent protocol amendment was enacted to shorten the duration of Tala treatment from continuous (28 out of 28 day) dosing to 21 out of 28 day dosing per cycle. Accrual is ongoing with plans to open this trial at two other centers in 2022.
Przespolewski: Jazz: Research Funding. Griffiths: Taiho Oncology: Consultancy, Honoraria; Boston Biomedical: Consultancy; Apellis Pharmaceuticals: Research Funding; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; Abbvie: Consultancy, Honoraria; Novartis: Honoraria; Alexion Pharmaceuticals: Consultancy, Research Funding; Takeda Oncology: Consultancy, Honoraria; Astex Pharmaceuticals: Honoraria, Research Funding. Thompson: Novartis/ Bristol-Myers Squibb: Research Funding. Elshoury: Bristol Meyers Squibb: Other: advisory board. Wang: AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Other: Advisory Board; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Novartis: Consultancy, Honoraria, Other: Advisory Board; Mana Therapeutics: Consultancy, Honoraria; Kura Oncology: Consultancy, Honoraria, Other: Advisory board, steering committee, Speakers Bureau; Kite Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Stemline Therapeutics: Consultancy, Honoraria, Other: Advisory board, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Advisory board; DAVA Oncology: Consultancy, Speakers Bureau; Rafael Pharmaceuticals: Other: Data safety monitoring committee; Gilead: Consultancy, Honoraria, Other: Advisory board; Daiichi Sankyo: Consultancy, Honoraria, Other: Advisory board; PTC Therapeutics: Consultancy, Honoraria, Other: Advisory board; Genentech: Consultancy; MacroGenics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal